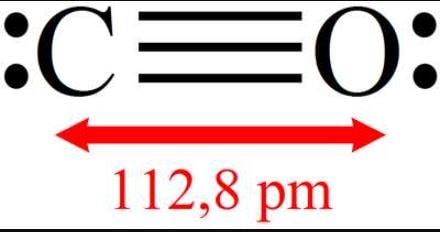
Carbon monoxide is an oxide of carbon. With the chemical formula CO, it is made up of one oxygen atom and one carbon atom.
Carbon Monoxide Poisoning
Carbon monoxide (CO), produced during poor combustion of organic fuels (wood, butane, coal, gasoline, fuel oil, natural gas, oil, propane … used for heating appliances, water production or the operation of engines: generator, for example), is a highly toxic gas even in small quantities.
Victims of acute poisoning require immediate and heavy hospital care such as respiratory assistance and oxygenation by hyperbaric chamber. These accidents can leave scars for life.
At a concentration of only 500 ppm (parts per million) of carbon monoxide in the air breathed in, there is the appearance of severe headaches, dizziness and a tendency to sleep announcing the onset of intoxication.
Muscular impotence and progressive paralysis appear when the individual is subjected to a concentration of 2000 ppm, followed by a coma if no help intervenes. In addition, death is rapid following an exposure of a few minutes to a dose of 5000 ppm. This death occurs when 66% of the hemoglobin has been transformed into carboxyhemoglobin.
Studies have made it possible to assess that in large cities, for example, the blood of the inhabitants contains 1 to 2% carboxyhaemoglobin. That of a smoker from 4 to 10% causing the heart to work harder in order to oxygenate the body.