Water is the source of all things, yet many important water-related processes on Earth, from the Earth’s environment to the functioning of human lungs, only take place on the surface of water droplets.
Decades ago, scientists predicted that water would separate into 2 liquids under supercooled conditions, and now new evidence shows that supercooled water can indeed change from one liquid to a denser liquid, undergoing a “liquid-liquid phase transition” instead of becoming solid or gas.
In 1992, researchers at Boston University first proposed that “supercooled water” has a hidden “liquid-liquid phase transition” state.
It’s just that water tends to freeze into solid ice rather than another liquid at low temperatures, so proving this phase transition is challenging, and the scientific community still has little knowledge about liquid-liquid phase transitions.
Now, using computer simulations, researchers from the University of Birmingham and the University of Rome have succeeded in distinguishing two liquid characteristics at the microscopic level, showing that water can change from one liquid to another, which is more dense.
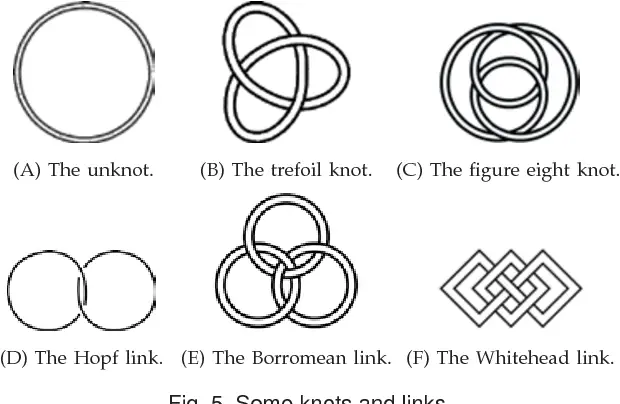
The team found that high-density liquid water molecules form topologically complexes, such as trefoil knots or Hopf links, that entangle in a pretzel-like shape, compared to low-density liquid water molecules that mostly form simple rings.
Furthermore, the knots are transient and exchange entanglements as the liquid environment changes, suggesting that the properties of liquid water at high pressure and low temperatures should be quite different from any sloshing water on the Earth’s surface.
Further understanding of the topological behavior of water and other liquids under supercooled conditions could help scientists unravel the state of materials in extreme environments, such as the depths of planets.
The new paper was published in the journal Nature Physics.