Researchers at Chalmers University of Technology have developed a new method for producing high-performance electrode materials using graphene, with this new method high-performance sodium batteries can be produced.
Batteries will cost much less
This new type of graphene makes it possible to store sodium, one of the most common and cheapest metal ions in the world. The results showed that the storage capacity could even match the current capacities of lithium-ion batteries.
Although lithium ions are well used for energy storage, lithium is an expensive metal, raising major concerns regarding its long-term supply and environmental problems.
Sodium is an abundant, inexpensive metal and is the main component found in seawater and table salt. This makes sodium batteries an excellent sustainable alternative to reduce our limited raw material needs.
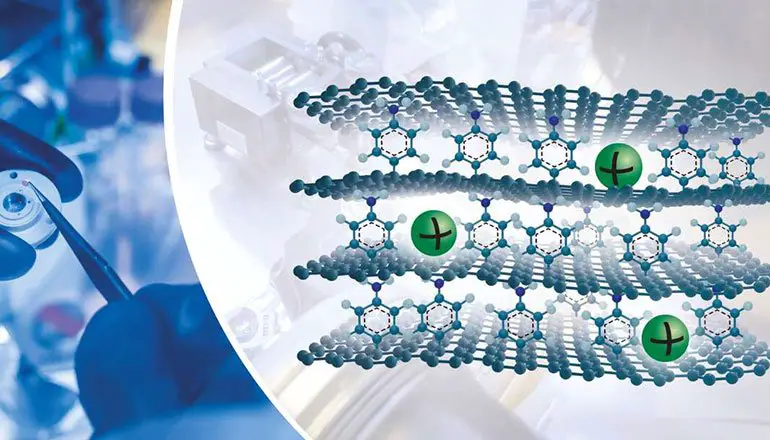
Ten times the energy capacity of graphite
Normally, the sodium intercalation capacity in graphite is 35 milliamperes/hour/gram (mA/h/g-1). This means that the interleaving capacity of lithium ion in graphite is less than one-tenth.
With this new graphene, the capacity increases to 332 milliamperes/hour/gram, approaching the value of lithium in graphite. The results also showed overall reversibility and increased stability of the cycle count.
The research is still at an early stage, but the results are pretty inspiring. The results mean that it is possible to form graphene layers in an ordered structure that adapts to sodium ions and compares them with graphite.
With the development of this technology, many different products from electric vehicles to mobile phones can be offered to users at lower costs in the future.