A team of Greek engineers, sponsored by the ESA, seeks to set up a method to detect the presence of reactive oxides in Martian and lunar soils, but also to exploit them in order to build oxygen farms for future assignments.
Solution by microorganisms
In 1976, the American Viking landers landed on the Martian surface with the objective, among other things, of determining the presence, past or present, of life on our neighboring planet.
Among the various on-board experiments, the results of one of them still remain controversial today: during the ” Labeled Release ” experiment, the robotic arm of the lander moistened a sample of Martian soil at using an aqueous solution of water enriched with a few organic molecules followed by carbon 14 (a radioactive isotope of carbon).
Thus, an increase in the concentration of radioactive CO 2 (or other gasescarbonaceous) could be interpreted as the result of the metabolization of organic molecules in the solution by microorganisms present in the soil sample.
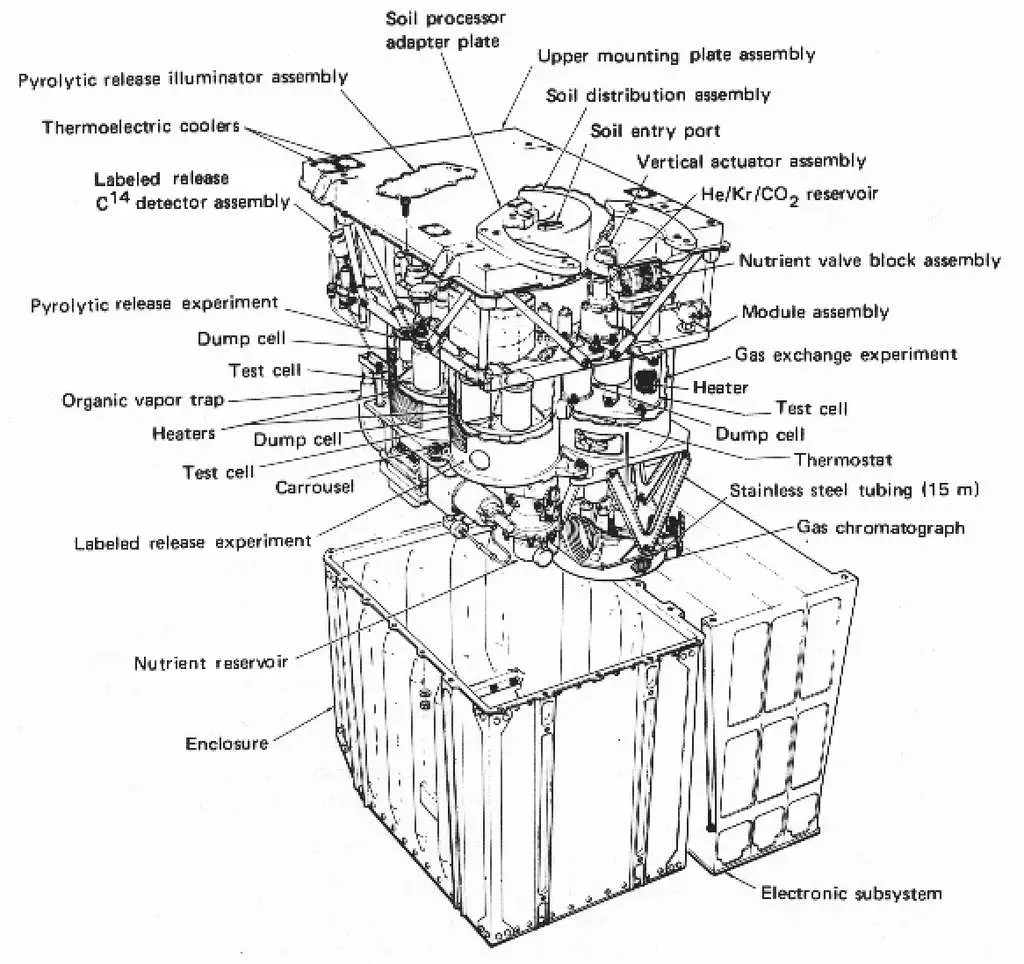 The laboratory module of a Viking lander. © Nasa
The laboratory module of a Viking lander. © NasaAnd the results of the experiments, still debated more than forty years later, were surprising to say the least: immediately after injection of the aqueous solution, a constant flow of radioactive carbon gases was measured at the outlet of the soil sample. Note that the experiment was carried out twice by the Viking 1 and 2 landers, one with a sample of regolith , the other with a sample of soil contained under a rock.
An abiotic origin?
But, for many exobiologists, these results do not constitute formal proof of biological activity: gas production was always measured after the sterilization of the sample (heated for 3 hours at 160°C).
To top it off, further experimentation from the Viking missions revealed no trace of organic compounds in the samples. Thus, many scientists agree that these gas emissions were probably due to abiotic chemical reactions . Some then point to the presence of highly reactive and oxygen -rich chemical compounds.in Martian soils: according to them, such compounds, such as superoxides, peroxides or even perchlorates, could react to the presence of liquid water to release oxidized ions. The presence of such compounds has already been confirmed on the surface of Mars.
A double-edged sword
Such reagents could have disastrous consequences for future manned missions: highly corrosive, these compounds could damage the machinery or attack the suits of astronauts, even burn their skin or damage their lungs . They would also have such an effect that they would be able to erase all traces of fossil life .
A team of scientists from the National Polytechnic University of Athens and the University of Patras then sought to develop a means of detecting and mapping the presence of these compounds on the Martian surface, by designing a device the size of a paperback book in which soil samples would be brought into contact with water in order to generate reactions (boosted under the action of catalysts ) similar to those observed by the Viking lander experiments.
In addition to reducing the surface area likely to contain traces of fossil life, mapping these compounds could be vital to allow future manned missions to avoid these potentially hazardous areas.
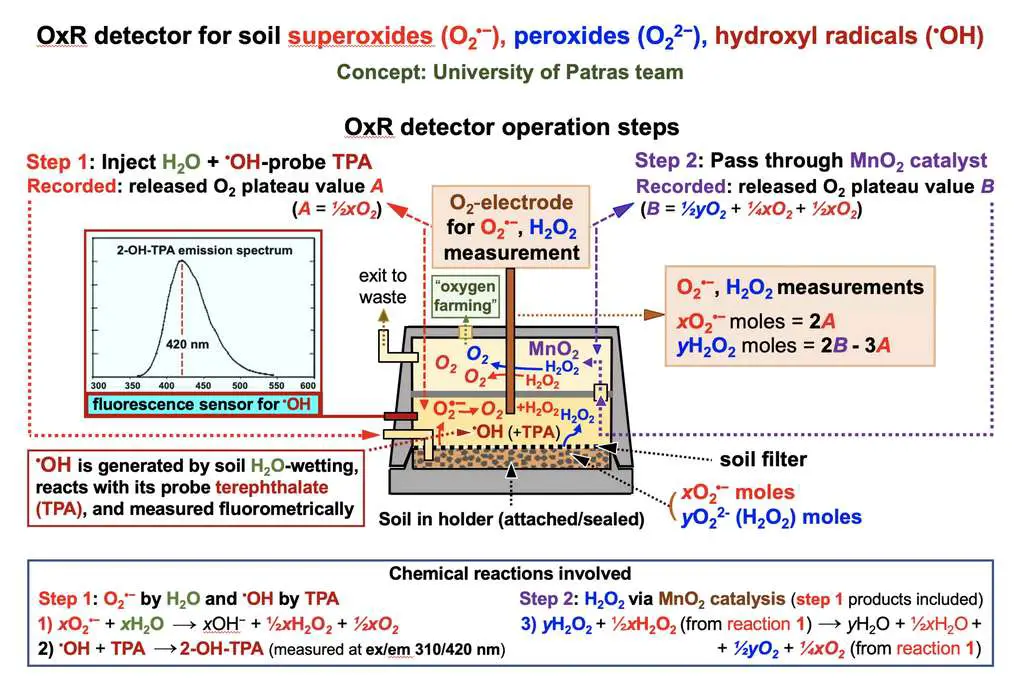 Reactive oxygen species detector concept. © National Polytechnic University of Athens and University of Patras
Reactive oxygen species detector concept. © National Polytechnic University of Athens and University of PatrasBut the scientists do not stop there: according to them, these soils could also be used to produce almost infinite quantities of oxygen, usable for future Martian bases. The project, sponsored by ESA , therefore also includes the design of a first reactor prototype to periodically extract oxygen from the Martian soil.
The team has, for the moment, only carried out tests using soil samples from the Mojave and Atacama deserts, with characteristics close to those of Martian soils ; but for the sake of accuracy, scientists are now looking to produce a synthetic Martian regolith, or to use Martian meteorites for their tests.